Research Highlights
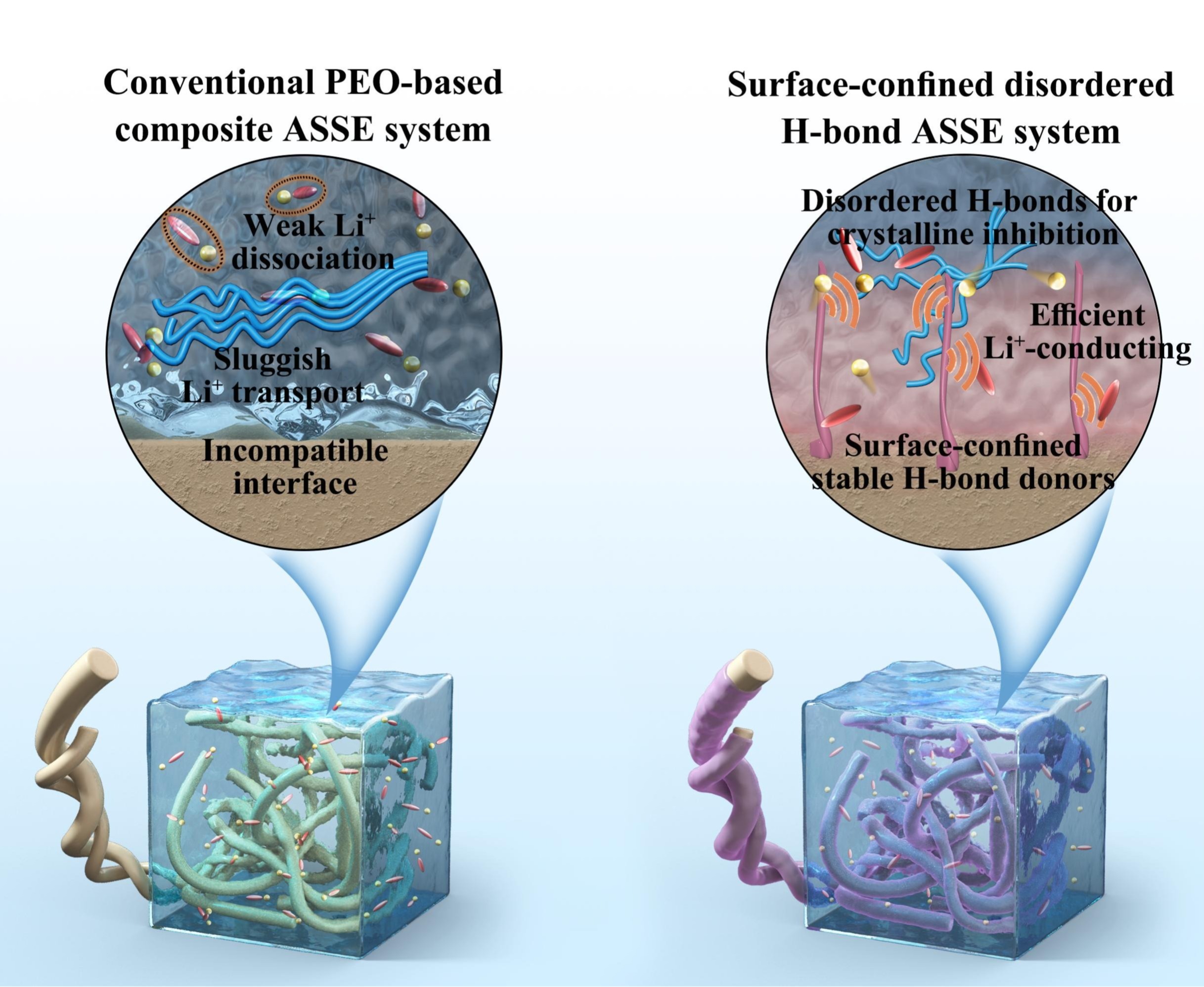
1. Surface-Confined Disordered Hydrogen Bond System Enables Stability Breakthrough in All-Solid-State Polymer Lithium Batteries
The Advanced Energy Storage Battery Technology Research Team at Fuzhou University developed a surface-confined disordered hydrogen bond system. By chemically anchoring 2-acrylamide-2-methylpropanesulfonic acid (AMPS) molecules onto glass fiber surfaces, they constructed a multi-donor-site hydrogen bond network, achieving a triple synergistic effect: 1)Crystallization Suppression: AMPS disrupts the ordered arrangement of PEO chains, forming a loose amorphous structure (XRD shows a 23% reduction in crystallinity); 2)Weakened Coordination: Hydrogen bonds reduce the electron cloud density of ether oxygen groups, weakening the strong EO-Li⁺ coordination (7Li NMR reveals a 0.15 ppm upshift in chemical shift); 3)Anion Anchoring: Sulfonic acid groups of AMPS form stable hydrogen bonds with TFSI⁻, inhibiting anion migration (Raman spectroscopy shows 97.2% of TFSI⁻ remains immobilized).
The rationally designed all-solid-state lithium battery (ASSLB) exhibits exceptional long-term cycling stability, retaining 87.5% capacity after 400 cycles at 65°C and 1.0 C, surpassing most reported polymer-based ASSLBs. This work provides a new paradigm for solid-state battery design: 1)Safety: Eliminates liquid electrolyte leakage risks and suppresses lithium dendrite growth (SEM confirms dendrite-free lithium anodes post-cycling) 2)Energy Density: A pouch cell achieves 98% capacity retention after 200 cycles at 0.5 C, outperforming existing counterparts.
This breakthrough, titled "Surface-Confined Disordered Hydrogen Bonds Enable Efficient Lithium Transport in All-Solid-State PEO-Based Lithium Battery", was published in《Angewandte Chemie International Edition》
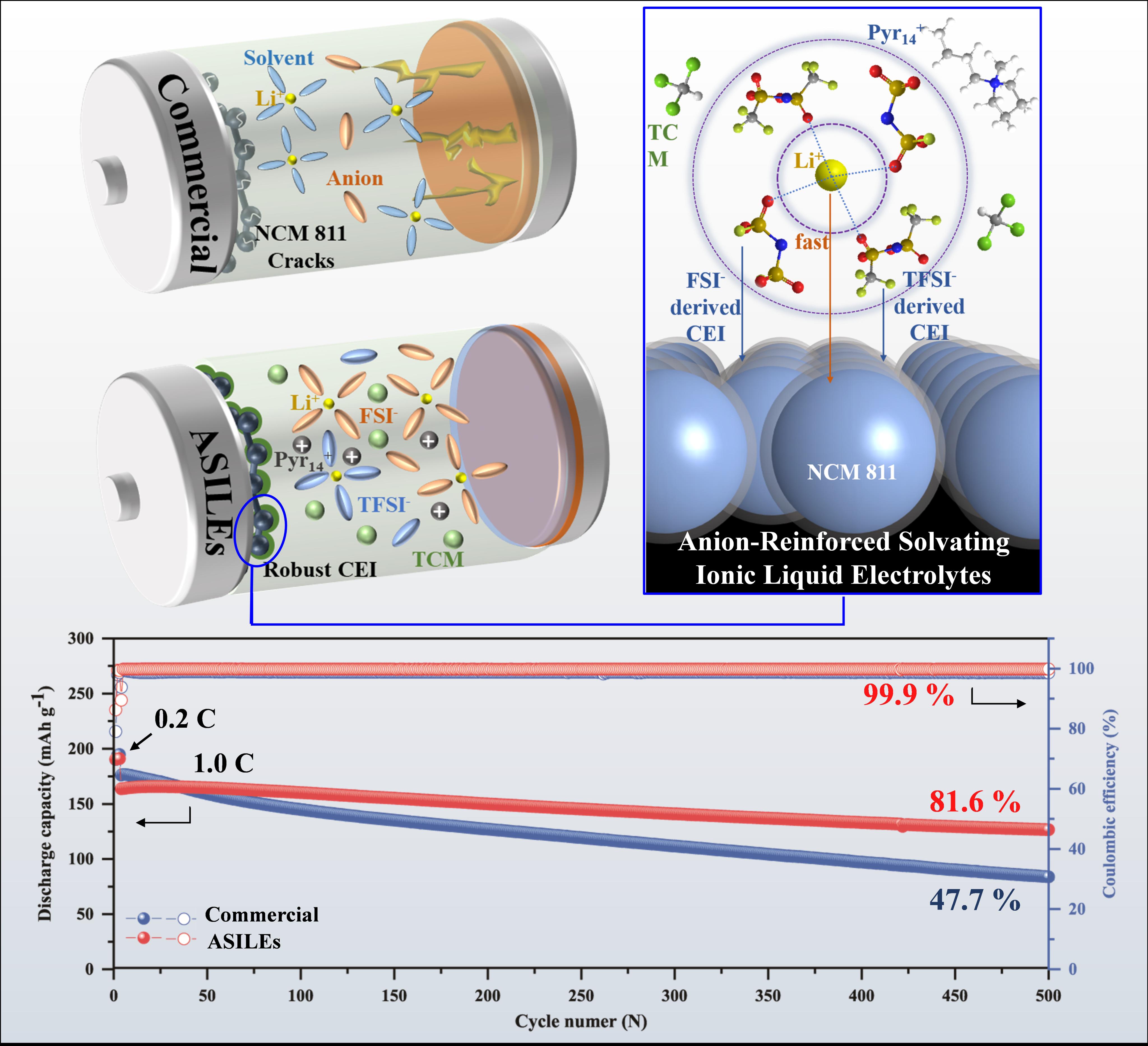
2. Strong Anion-Solvated Ionic Liquid Electrolyte Enables Stable High-Nickel Cathode in Lithium-Metal Batteries
To address the incompatibility between commercial carbonate electrolytes and high-energy lithium-metal batteries, Fuzhou University’s Advanced Battery Technology Team developed a dual-anion (FSI⁻ and TFSI⁻) reinforced ionic liquid electrolyte. This electrolyte combines the high ionic conductivity of traditional organic electrolytes with the superior interfacial stability and non-flammability of ionic liquid-based electrolytes.
The Li/NCM811 battery using this electrolyte demonstrates: 1)High initial specific capacity (203.0 mAh/g at 0.1 C); 2) Outstanding capacity retention (81.6% after 500 cycles at 1.0 C); 3) Exceptional average Coulombic efficiency (99.9% after 500 cycles at 1.0 C). Moreover, an ampere-hour-level Li/NCM811 pouch cell achieves an energy density of 386.0 Wh/kg, confirming the electrolyte’s practicality. This study offers a foundational strategy for designing advanced ionic liquid electrolytes, paving the way for high-safety, high-energy-density lithium-metal batteries.
The work, titled "Anion-Reinforced Solvating Ionic Liquid Electrolytes Enabling Stable High-Nickel Cathode in Lithium-Metal Batteries", was published in the prestigious journal 《Advanced Materials》.